44 structure function claims on dietary supplement labels
Dietary Supplement - an overview | ScienceDirect Topics Dietary supplements may make structure-function claims that focus on nonnutritive or nutritive effects. The FDA reaffirmed this point about structure-function claims for conventional foods in the September 23, 1997 (62 FR 49859), final rule entitled "Food Labeling: Nutrient Content Claims, Health Claims, and Statements of Nutritional ... ''Structure/Function'' Claims in Dietary Supplement Labeling Enactment of the Dietary Supplement Health and Education Act of 1994 (DSHEA) permitted manufacturers of dietary supplements to make structure/function' claims on labels, describing the helpful...
Label claims for foods and supplements: a review of the ... Structure/function claims describe an effect of a product on body structure or function, and whereas these claims must be truthful and not misleading, they are not subject to premarket scientific review and approval. Nutrient content claims describe the level of a nutrient in a food or supplement and require FDA approval.

Structure function claims on dietary supplement labels
Background Information: Dietary Supplements - Consumer The label of a dietary supplement or food product may contain one of three types of claims: a health claim, nutrient content claim, or structure/function claim. Health claims describe a relationship between a food, food component, or dietary supplement ingredient, and reducing risk of a disease or health-related condition. An examination of structure-function claims in dietary ... Dietary supplement advertising cannot claim a causal link between the product and the treatment, prevention, or cure of a disease unless manufacturers seek approval from the FDA for a health claim. Manufacturers can make structure-function (S-F) claims without FDA approval linking a supplement to a … Dietary Supplement Label Claims: What's Allowed? What's ... Any kind of promotion or labeling must be in accordance to the rules laid out by the Dietary Supplement Health and Education Act of 1994 (DSHEA) and corresponding implementing regulations issued by the FDA. Types of Claims. There are three main types of claims that can be made for Dietary Supplements: Health claims . Structure/function claims
Structure function claims on dietary supplement labels. Structure/function claims in dietary supplement labeling ... 1. Food Drug Law J. 1999;54(1):35-42. Structure/function claims in dietary supplement labeling: not all of these claims need to be submitted to FDA and accompanied in labeling by the DSHEA disclaimer. Structure/Function Claims in Dietary Supplement Labeling ... One important means of promoting dietary supplements is the inclusion, in label ing, of claims about the impact on the structure or function of the human body. These claims are known as "structure/function" claims. The author has observed the evolu tion of the Food and Drug Administration's (FDA's) regulation of dietary supplement STRUCTURE/FUNCTION CLAIMS ON DIETARY SUPPLEMENTS - USFDA Blog The manufacturers or labellers often describes the role of a product or an ingredient characteristic to affect normal structure or functions in humans either by statement or pictures on dietary supplements. They are called as structure/function claims if and only if it is not linking to a disease in the context. Constructing structure and function claims | Natural ... There are three main types of label claims that marketers may make about their supplement products 1) Structure/function claims (and the related claims of general well-being and claims related to a nutrient deficiency disease) 2) Health claims (and qualified health claims) 3) Nutrient-content claims
Structure/Function Claim Notification for Dietary ... The law states that a dietary supplement may bear certain statements on its label or in its labeling if the claim (s) meets certain requirements, if the entities making the claims have... Structure Function Claims | Build Your Business, In Compliance On the other hand, certain claims, called "structure function claims", may be made about dietary supplement products and, if done correctly, will not cause these products to be subject to heightened regulation. More information about Structure Function Claims and FDA regulations may be accessed here. OIG Report Finds that Structure/Function Claims on Dietary ... The OIG's report entitled Dietary Supplements: Structure/Function Claims Fail to Meet Federal Requirements analyzed structure/function claims that manufacturers may use on dietary supplement labels to promote their products.. A structure/function claim describes how an ingredient in the dietary supplement will affect or maintain a structure or function in the human body. Label Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims Structure/Function Claims | FDA Mar 07, 2022 · The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of dietary... Dietary Supplements: Structure/Function Claims Fail To ... We analyzed structure/function claims for a purposive sample of 127 dietary supplements marketed for weight loss or immune system support. We reviewed the claims to determine the extent to which they complied with FDA regulations. We reviewed substantiation provided by manufacturers to describe the quantity and nature of the evidence. Structure Function Claims - Dietary Supplement Experts The Dietary Supplement Health and Education Act of 1994 (DSHEA) added section 403 (r) (6) to the Federal Food, Drug, and Cosmetic Act (FD&C Act). This section of the law states that a dietary supplement may bear structure/function claims and provides their requirements. Further, 21 CFR 101.93 also restates these requirements.
FDA Labeling Guidance: Making Structure/Function Claims ... The FDA permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or ...
Structure/Function Claims Basics - Dietary Supplement Experts Structure/function claims describe the role of a nutrient or dietary ingredient intended to affect the structure or function of humans, or characterize the documented mechanism (s) of action by which a nutrient or dietary ingredient acts to maintain such structure or function. Importantly, they cannot be disease claims.
FDA Labeling Guidance: Making Structure/Function Claims ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in humans" or characterize the "documented mechanism" by which the nutrient acts to maintain such structure or function, but do not claim to "diagnose, …
Structure/Function Claim Notification for Dietary Supplements Office of Dietary Supplement Programs (HFS-810) Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740-3835 Contact the Office of Dietary...
The Basis of Structure/Function Claims of Nutraceuticals Structure/function claims are labeling claims that can be used to describe the potential effects of a dietary ingredient or similar substance on the structure or function of the human body. This category of claims was created by legislation contained in the Dietary Supplement Health and Education Act.
PDF Permissible vs. Impermissible Structure/Function Claims ... Structure/Function Claims for Dietary Supplements. 2 THE BASICS: ... the heart symbol on product label and labeling is an impermissible heart disease prevention claim.) 14 ... No more than thirty (30) days after a supplement bearing a structure/function claim is marketed, the manufacturer, packer, or distributor of the
Dietary Supplement Labeling Guide: Chapter VI. Claims | FDA you must use a disclosure statement when you make a nutrient content claim and your food (including dietary supplements) contains one or more of the following nutrients in excess of the levels...
Federal Preemption of 'Structure/Function' Claims on ... By definition, a structure/function claim "describes the role of a nutrient or dietary ingredient intended to affect the structure or function in humans [or] characterizes the documented mechanism...


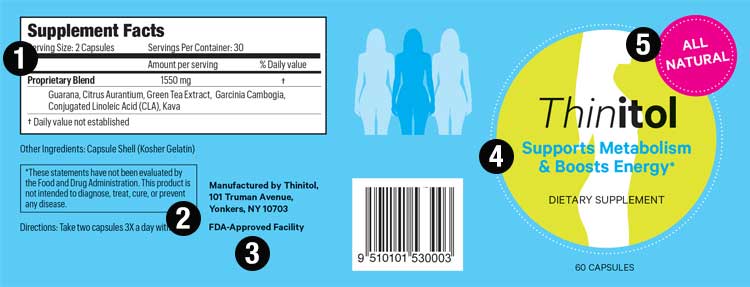


Post a Comment for "44 structure function claims on dietary supplement labels"