43 fda guidance use of symbols on labels
PDF NDA 215272 NDA APPROVAL - accessdata.fda.gov content of labeling [21 CFR 314.50(l)] in structured product labeling (SPL) format using the FDA automated drug registration and listing system (eLIST), as described at FDA.gov. 1 . Content of labeling must be identical to the enclosed labeling (text for the Prescribing Information and Patient Package Insert,) as well as annual reportable Recognized Consensus Standards - accessdata.fda.gov Recognized Consensus Standards. This document specifies symbols used to express information supplied for a medical device. This document is applicable to symbols used in a broad spectrum of medical devices, that are available globally and need to meet different regulatory requirements. These symbols can be used on the medical device itself, on ...
Draft Guidance for Industry and FDA Staff; Use of Symbols ... This document provides guidance on the use of selected symbols in place of text to convey some of the information required for in vitro diagnostic devices (IVDs) intended for professional use by FDA's labeling requirements for IVDs. This draft guidance is not final nor is it in effect at this time. DATES:
Fda guidance use of symbols on labels
PDF Use of Symbols in Labeling - fda.gov FDA assumes that the labeling of each uniquely labeled medical device can communicate the same information using -alone standsymbols or symbols with adjacent explanatory text. FDA further assumes... CFR - Code of Federal Regulations Title 21 - Food and Drug ... Subpart A - General Labeling Provisions § 801.1 - Medical devices; name and place of business of manufacturer, packer or distributor. § 801.3 - Definitions. § 801.4 - Meaning of intended uses. § 801.5 - Medical devices; adequate directions for use. § 801.6 - Medical devices; misleading statements. § 801.15 - Medical devices; prominence of required label statements; use of symbols in ... Final rule on use of symbols on labeling - Symbols without ... Jun 14, 2016. #4. Jun 14, 2016. #4. FDA issues final rule on use of symbols in labeling. Today the Food and Drug Administration (FDA) issued a final rule "Use of Symbols in Labeling" allowing the use of stand-alone symbols in medical device labeling, without adjacent explanatory text.
Fda guidance use of symbols on labels. Device Labeling | FDA Labeling regulations pertaining to medical devices are found in the following Parts of Title 21 of the Code of Federal Regulations (CFR). General Device Labeling - 21 CFR Part 801 Use of Symbols -... PDF Use of Symbols on Labels and in Labeling of In Vitro ... Guidance for Industry and FDA Staff Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use Document issued on: November 30, 2004 The draft of this document was issued on October 28, 2003. The information collection provisions in this guidance have been approved under OMB control number 0910-0553. Safety Considerations for Container Labels and ... - fda.gov 5 Although this guidance focuses on container labels and carton labeling considerations for drug and biological products, the recommendations are also relevant to combination products regulated by ... Guidance for Industry and Food and Drug Administration ... The guidance document entitled "Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use" provides guidance on the use of those recognized symbols. FDA announced the availability of the level 1 draft guidance document in the Federal Register of October 28, 2003 ( 68 FR 61449 ).
Packaging and labelling | Food Standards Agency How to display mandatory information on packaging and labels. A minimum font size applies to mandatory information which you must print using a font with a minimum x-height of 1.2mm. If the largest surface area of packaging is less than 80cm squared, you can use a minimum x-height of 0.9mm. Mandatory details must be indicated with words and ... Use of Symbols in Medical Device Labeling- FDA's Final ... The rule finalizes the FDA's 2013 proposed rule and affects biologics, IVDs, and medical devices regulated by 21 CFR Parts 660, 801, and 809. The revision allows for the inclusion of symbols in labeling without adjacent explanatory text (referred to as "stand-alone symbols"), so long as certain requirements are met. Advertising FAQ's: A Guide for Small Business | Federal Trade ... Apr 04, 2001 · The FDA handles most matters regarding food labels. For guidance on how the FTC evaluates claims made in food ads, ask the FTC for the Enforcement Policy Statement on Food Advertising. For information about product labeling, visit the FDA's website at or call the FDA Inquiry Line, 1-888-INFO-FDA Economic Impact Analyses of FDA Regulations May 06, 2022 · The Food and Drug Administration conducts economic analyses of all important proposed and final regulations. Each economic analysis includes an assessment of the costs, benefits, and cost ...
Federal Register :: Use of Certain Symbols in Labeling the food and drug administration (fda) is proposing to revise medical device and biological product labeling regulations to explicitly allow for the inclusion of stand-alone graphical representations of information, or symbols, if the symbol has been established as part of a standard developed by a nationally or internationally recognized … CFR - Code of Federal Regulations Title 21 - Food and Drug ... (c) (1) (i) All words, statements, and other information required by or under authority of the act to appear on the label or labeling for a device shall appear thereon in one or more of the... FDA Issues Final Rule on Use of Symbols in Labeling - Lexology the final rule allows symbols established in a standard developed by a standards development organization to be used in medical device labeling without adjacent explanatory text if (1) the standard... Prescription drug - Wikipedia In the United States, the Federal Food, Drug, and Cosmetic Act defines what substances require a prescription for them to be dispensed by a pharmacy. The federal government authorizes physicians (of any specialty), physician assistants, nurse practitioners and other advanced practice nurses, veterinarians, dentists, and optometrists to prescribe any controlled substance.
The FDA considers a "healthy" food label The FDA itself is in the process of updating its definition, which dates back to 1994. Other concerns are that such a label could be of dubious value, used too liberally by food makers, or seen by consumers as a product endorsement by the FDA. Nutritionists make the point that a balanced diet matters more to health than any individual food.
PDF Use of Symbols on Medical Device Labels - FDA Requirements In all such cases, we use EN 980/ISO 15223 symbology on the label, with English-only subtext for each symbol in a small but readable font size. We have been doing this for a number of years now, and have had no objection from US FDA, our NB, our Authorized Representative, authorities in Europe, or end users. adv_webdev24th June 2011 04:45 PM
FDA Issues Final Rule Permitting Use of Symbols on Device ... Three years ago, FDA proposed a rule that would permit device manufacturers to use stand‑alone symbols on device labeling. In the meantime, this issue has continued to fester. So it is good news that FDA finalized the rule on June 15, 2016. Under the final rule, device manufacturers may use symbols on device labeling in one of the following ways:
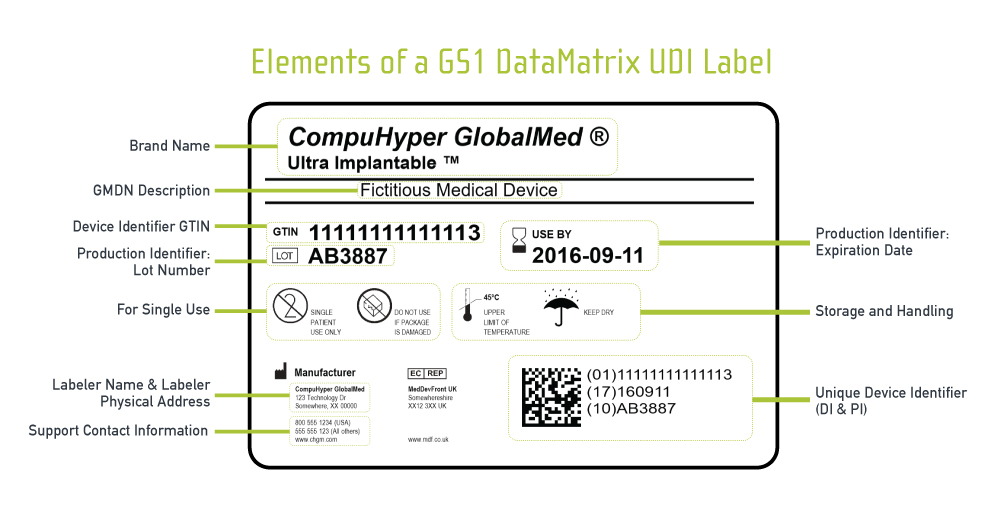
PDF Symbols Commonly Used in Medical Device and Packaging Labeling Use of the "Rx only" symbol as an alternative to the prescription device labeling statement. FDA Guidance Alternative to Certain Prescription Device Labeling Requirements N/A 2. Alarm to indicate an alarm on a control equipment IEC 60601‐1‐8 Table C1, No. 1 3. Alarm Reset To identify the control for ALARM RESET.


Post a Comment for "43 fda guidance use of symbols on labels"